May 13, 2019
Improving Stability and Secretion of Antibodies
Life Sciences, Immunology
- Improvement of the biophysical properties of antibodies and enhancement of antibody secretion by imitating the stabilizing structural elements of shark antibodies
- CL domain is modified by a conservative exchange of amino acids at two positions
- Compared to the wild type CL, the melting point of the modified CL domain is almost 10°C higher and its stability against urea-induced denaturation is also markedly increased
Your contact
Dr. Katharina Stoiber
- E-Mail:
- kstoiber@baypat.de
- Phone:
- +49 (0) 89 5480177 - 40
- Reference Number:
- B73076
Factsheet
Download Tech Offer (PDF)Challenge
The constant domain of the antibody light chain (CL) is essential for both folding of the heavy chain CH1 domain and consequently also for quality control of correct antibody assembly. Since the CH1 domain can only adopt its native conformation after association with the CL domain, alterations in CL may have an impact on the efficiency of assembly and secretion of antibodies.
Innovation
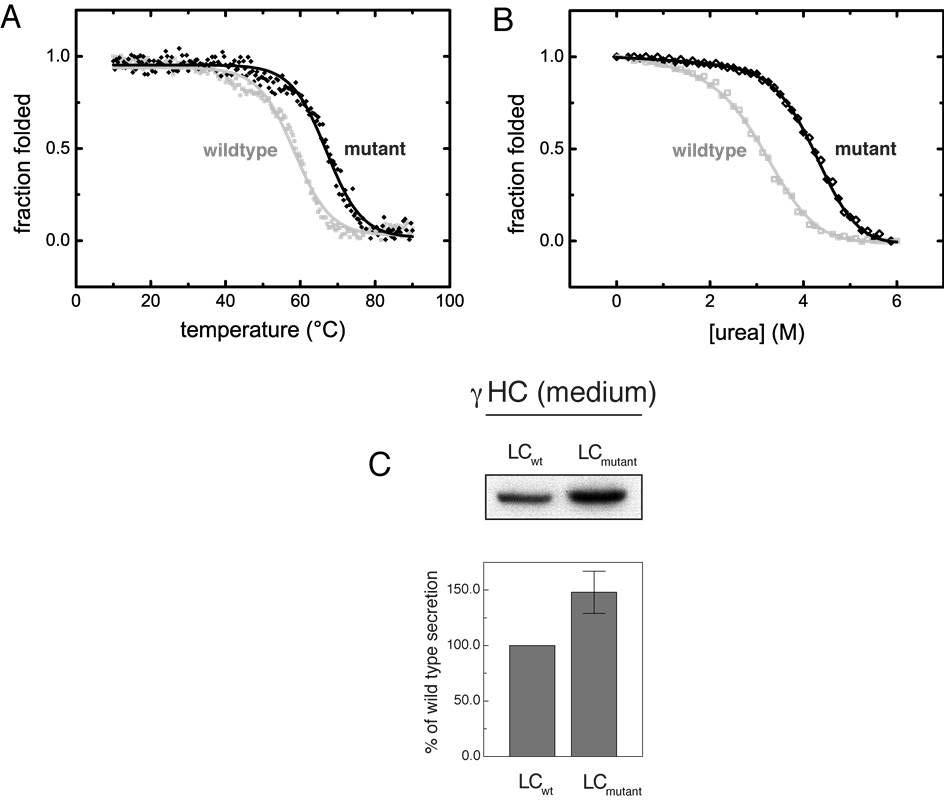
The present invention improves the biophysical properties of antibodies and results in a strongly increased secretion. Following the stabilizing structural elements of shark antibodies, the CL domain is modified by an conservative exchange of amino acids at two positions. This leads to the formation of an internal salt bridge and an extended hydrophobic core. Compared to the wild type CL, the melting pointof the modified CL domain is almost 10°C higher and its stability against urea-induced denaturation is also markedly increased (see Figure below).
Notably, when light chains comprising this optimized CL domain were co-expressed with Ig heavy chains, a significant increase in the assembly and subsequent secretion of complete IgG antibody mo-lecules from mammalian cells was observed. Although this technology was established using IgG, it is applicable to all classes of antibodies because of the ubiquitous presence of CL in all immunoglobulin classes. Furthermore, this invention will also be applicable for the optimization of other constant do-mains besides CL.
Commercial Opportunities
USP: Antibodies with significantly higher stability and strongly increased secretion
Development Status
Proof of concept in vitro
References
-
Reference
Feige et al., PNAS 2014 Jun 3;111(22):8155-60