Jun 25, 2019
NF-κB-activated dendritic cells
Life Sciences, Immunology
- that has very little adverse effects and overcomes the limitations of DC based immunotherapy approaches
- DCs activation is mediated by via mRNA transfection
- Activated DCs lead to strong expansion of memory T cells and are much more stable than other DC vaccination products
Your contact
Dr. Katrin Bercht
- E-Mail:
- kbercht@baypat.de
- Phone:
- +49 (0) 89 5480177 - 16
- Reference Number:
- B71259
Factsheet
Download Tech Offer (PDF)Challenge
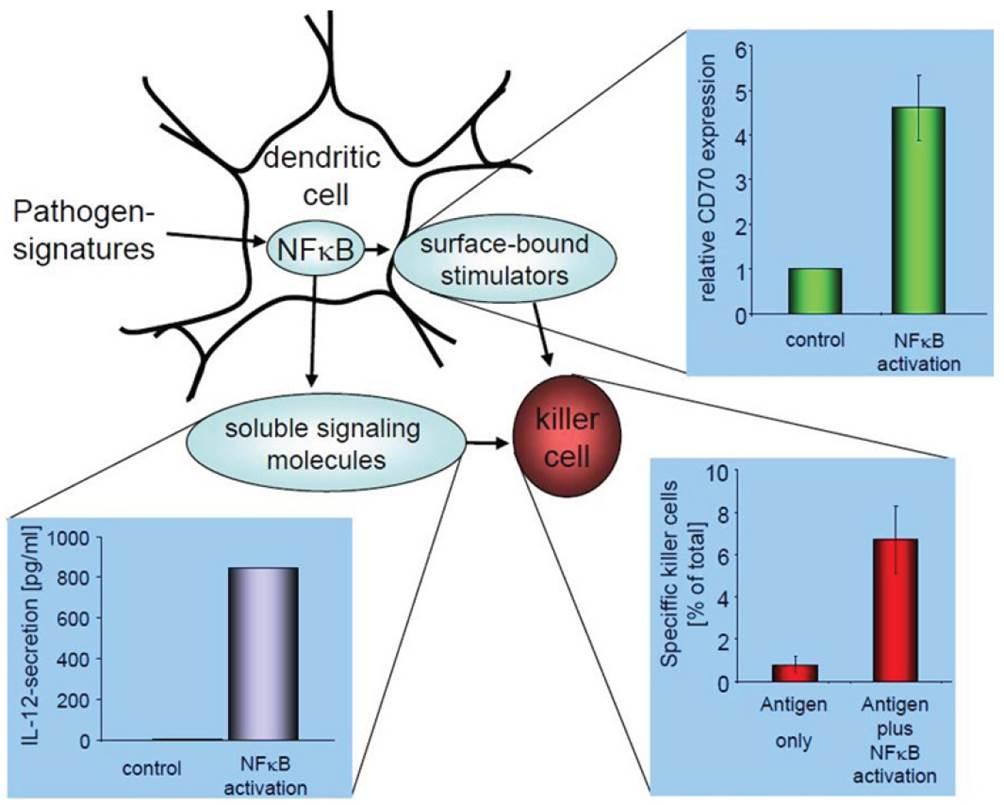
Dendritic cells (DCs) as antigen presenting cells of the mammalian immune system characteristically have the ability to activate T cells which can be exploited for cancer immunotherapy. Although immune responses are clearly induced by the present DC vaccines, clinical responses lack behind since cytokine-treated DC, which are normally used, are limited in their IL-12 production - a cytokine critical for immunologic memory.
Innovation
The present technology overcomes these limitations. Here, DCs are activated by triggering their NF-κB-pathway via mRNA transfection. This results in constitutively active and stabilized DCs that secrete enhanced amounts of immune-stimulatory cytokines including IL-12p70. Furthermore, these DCs comprise an intrinsic activation signal and therefore stay active longer. This is not only crucial since DCs need time to reach lymphatic tissue to do their job. Most importantly, cytotoxic T cells induced by these designer DCs combine a memory-like phenotype and a markedly increased secondary expandability with a high lytic capacity. This offers cancer patients a DC-vaccination treatment that has very little adverse effects and can be combined with other new immunotherapeutic approaches.
Commercial Opportunities
NF-kB-activated DCs
- show increased IL-12 production and capacity to repetitively stimulate and expand antigen-speci-fic CTLs compared to other DC based immunotherapies
- lead to strong expansion of effector T cells (memory T cells)
- are much more stable compared to other DC vaccination products
- can be applied to different sorts of cancers
- can be combined with other immunotherapeutic approaches such as small molecule inhibitors or checkpoint blockade inhibitors to improve benefit
Development Status
Preclinical testing is at a stage that allows starting a phase I clinical trial.
References
-
Reference
Pfeiffer et al., Eur. J. Immunol. 2014. 44: 3413–3428